The coronavirus broke out in Wuhan City, China in late 2019. Within several months, this epidemic has evolved into a global pandemic.
As people in over 200 countries and regions are combating the disease and searching for a cure, we would like to present a holistic view of what we can learn from the pandemic: about our society, modern science, and culture as well as history.
It is our hope that this four-part series will help our readers understand the pandemic would not have happened without continued misleading information from the Chinese Communist Party (Part 1). We also examine theories of where the coronavirus started (Part 2) and how it started (Part 3).
Understanding the pandemic in the context of culture and history (Part 4), on the other hand, offers clues of how to reevaluate our principles and moral obligations while preparing for the next chapter in history.
Below is an outline of the series:
Part 1: Timeline and Analysis
Chapter 1: Cover-ups of the Outbreak in China
Chapter 2: Will Such Tragedies Happen Again?
Part 2: A Mysterious Virus — Where Did It Start?
Chapter 3: US-origin Theory
Chapter 4: China-origin Theory
Part 3: A Mysterious Virus — How Did It Start?
Chapter 5: Man-made Theory
Chapter 6: Natural-origin Theory
Part 4: Rethinking Modern Science and Returning to Traditional Values
Chapter 7: The CCP Poses An Unprecedented Challenge to Humanity
Chapter 8: Reflection on Ancient Wisdom
*****
(Continued from Part 2)
Part 3: A Mysterious Virus — How Did It Start?
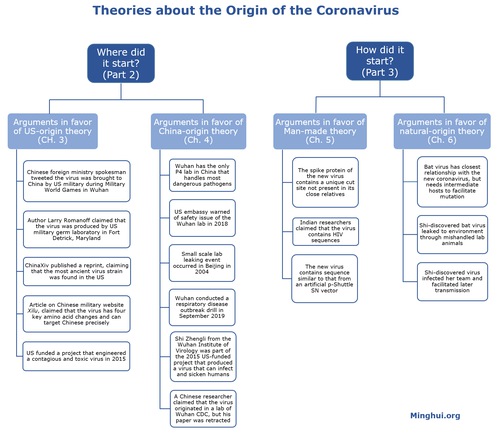
As indicated in Part 2, several theories have been circulated about the origin of the coronavirus (see image below).
One type of theory concerns where the virus started, and the other examines how the virus started.
As shown in the following image, we cover where the virus started in Part 2 and examine arguments in favor of the competing U.S.-origin (Chapter 3) and China-origin (Chapter 4) theories.
Part 3 looks into how the virus started and presents arguments in favor of man-made theory (Chapter 5) and natural-origin theory (Chapter 6).
Chapter 5: Man-made Theory
So far, the main arguments that support the man-made theory about the origin of the virus are centered around the sequence of its spike protein (S-protein), as well as a piece of sequence insertion that appeared to come from an artificial vector used for DNA manipulation.
1. The spike protein of the new virus contains a unique cut site not present in its close relatives.
Scientists have found that the S-protein of the new coronavirus has a special fragment of sequence that can be cleaved by a special protein in the host cell. After the sequence was cut by the protein called furin, the virus gains the ability to enter the host cell and infect multiple organs.
Some people argued that the furin cleavage site in the virus is unique and hasn’t been found in its close relatives of other coronaviruses, but some scientists also point out that such sequence does exist naturally in other viruses, including some coronaviruses not directly related to the new virus.
While one could argue that the insertion of the furin cut site to the virus is through genetic manipulation, as it’s a well-established bioengineering process, one can’t completely rule out the possibly that the virus takes up the sequence from the environment itself, given the fact that coronavirus is a RNA virus, which isn’t stable and is constantly mutating and taking in sequences from the environment (a process called genetic recombination).
As a result, the furin cut site alone isn’t enough to conclude that the virus was a product of lab manipulation.
2. Indian researchers claimed the virus contains HIV sequences
There are also some other arguments about the virus’ S-protein containing HIV sequence. A preprint of an article from Bishwajit Kundu and others at the University of New Delhi was published in bioRxiv in late January.
The Indian team noticed four insertions in the virus S-protein are unique. They are not present in other coronaviruses, and amino acids are actually identical or similar to proteins in HIV. The Wuhan virus “is unlikely to be fortuitous in nature,” wrote the authors. Two days later, however, the Indian team withdrew this paper.
3. The new virus contains sequence similar to that from an artificial p-Shuttle SN Vector
Another argument that claims the Wuhan virus to be a lab-engineered strain was based on an article published on January 30, 2020, by James Lyons-Weiler, who formerly worked at the University of Pittsburgh as a bioinformatician.
In his article, Lyons-Weiler wrote that he observed a gene sequence in the Wuhan virus being 67% identical to the p-Shuttle SN Vector, which has been used in many labs to produce SARS vaccine. He then speculated that the novel coronavirus was a man-made virus used for SARS vaccine research.
Another researcher, Steven Salzburg, a computational biologist and professor at Johns Hopkins School of Medicine, searched the virus sequence against the database on NCBI (National Center for Biotechnology Information), only to see the top hits were sequences from other bat coronavirus, but not the vector.
Salzburg argued that if the virus sequence did come from the vector, it would be “near-identical,” not [67%] “distantly related.”
Chapter 6: Natural-origin Theory
Given above rebuttal to the man-made theory, no solid evidence could support the argument that the virus was actually generated in a lab. Many scientists agree that the new coronavirus was probably a naturally occurring virus that originated in bats.
1. Bat virus has closest relationship with the new coronavirus, but needs intermediate hosts to facilitate mutation
On February 3, 2020, Shi Zhengli published a paper on the prestigious journal Nature, titled “A pneumonia outbreak associated with a new coronavirus of probable bat origin.”
In this paper, Shi reported that through whole genome sequencing, her team has identified a bat coronavirus named RaTG13, sharing 96.2% identity with the novel coronavirus. This is the closest strain to the novel coronavirus reported so far.
The 96.2% similarity does not mean that the bat virus would directly infect humans and be responsible for the current pandemic. According to Trevor Bedford, a bioinformatics specialist at the University of Washington, it would usually take 25-65 years for the bat virus to mutate enough to become 100% identical to the current coronavirus.
However, the new coronavirus broke out only a few months ago, and there was not enough time for the bat virus to eliminate the 3.8% difference (=100% – 96.2%) and become the coronavirus if it had directly infected humans.
The only possibility for the bat virus to quickly mutate into the coronavirus was through intermediate hosts. In other words, if the bat virus had infected an intermediate host, which then spread the virus to humans, that’d greatly accelerate the mutation speed. Richard Ebright of Rutgers University argued that “the mutation rate may have been different as it passed through different hosts before humans.”
The quest is on to identify the intermediate host(s). In previous zoonotic outbreaks (diseases spread from animal to human), both the 2003 SARS in China and the 2012 MERS in Saudi Arabia were found to result from bat viruses that used palm civets and camels as intermediate hosts, before jumping back to human and causing diseases.
By the time the virus broke out in Wuhan in the winter of 2019, bats have already gone into hibernation and there were no bats sold at the wet market. So it’s possible that the virus has been around in the environment for months or even longer and has gone through a comprehensive mutation process before developing the deadly traits.
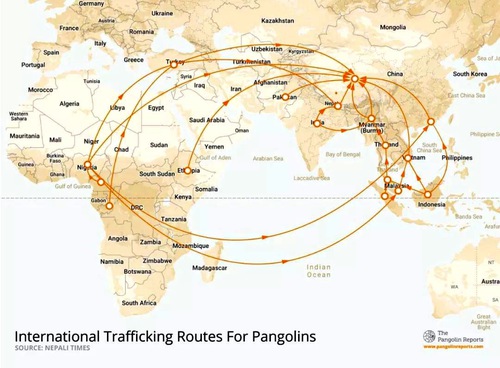
Several candidates came up in the search of the intermediate host, including mink, ferrets and even turtles. Although the list was narrowed down to pangolin as a hot contender, it soon proved to be impossible too.
The pangolin lives in a warm environment and has to stay in a subtropical environment unlike Wuhan. Its diet is limited to ants and termites. It also has a weak digestive and respiratory system. Pangolins get sick easily and the illness is often lethal. Because of these reasons, pangolin is not suitable for captive breeding. In fact, China does not approve its trading, so pangolins come from smuggling and very few of them are alive.
Had pangolins been an intermediate host, the first patients would have been smugglers. The outbreak would have also spread in various locations, since Wuhan is not a distribution center of the smuggling.
Also because of their weak respiratory system, pangolins get sick easily upon coronavirus infection. If they are sick, they could die before spreading disease.
Researchers at South China Agricultural University at one point announced that their sequencing result of the virus isolated from pangolins share 99% similarity to the new virus, but that turned out to be miscommunication within the team and the actual genome homology between the pangolin virus and the Wuhan virus is only 90%.
In 2003 SARS, civet was determined to be the intermediate host for the bat virus to make the jump to humans, after Shi Zhengli found a virus on civet that was 99.8% identical to SARS. So the 90% homology in pangolins was not sufficient to determine pangolins were the ultimate intermediate host that the scientists are looking for regarding the new coronavirus.
Even though there is no definitive answer to what could be an intermediate host for the coronavirus, tracing back Shi Zhengli’s journey to find the source of the 2003 SARS origin provides a clue of how the bat virus she discovered years ago may have eventually resulted in the coronavirus.
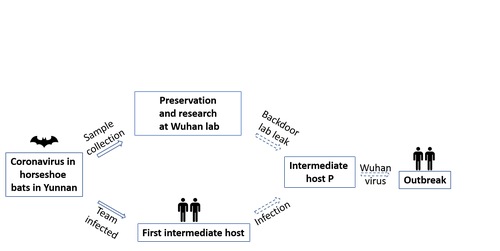
It took Shi 7 years to eventually find the very first origin of the SARS, very likely to the be RaTG13 strain, in a bat cave in Kunming City, Yunnan Province. But that begs the question of how the bat virus she found traveled 1,000 miles from Kunming to Wuhan, before jumping onto the unknown intermediate host?
We present two possible routes (see image below): first, the virus was brought back by Shi’s team and leaked into the environment through mishandled lab animals; second, Shi’s team members were infected with the bat virus and unknowingly became the first generation of intermediate host before transmitting the virus to the next host (animals) and jumping back to humans and caused the pandemic.
2. Shi-discovered bat virus leaked to environment through mishandled lab animals
While many people suspect that the virus was leaked from the Wuhan virology lab and it’s also widely known that Chinese research labs often have loose safety management, it isn’t that easy for a virus to be leaked from the lab after all.
First of all, BSL-4 labs are rare and they are designed for highly dangerous microbes. They are used to handle fatal microbes that do not have treatment or vaccines such as Ebola viruses.
A person is required to change clothing before entering and shower upon exiting. All materials are also decontaminated before exiting. In addition, one must wear appropriate personal protective equipment, as well as a full body, air-supplied, positive pressure suit.
A BSL-4 laboratory is extremely isolated—often located in a separate building or in an isolated and restricted zone of the building. The laboratory also features a special supply and exhaust air, as well as vacuum lines and decontamination systems.

Moreover, a BSL-4 lab operator is often accompanied with colleagues with the lab under video surveillance. This makes it almost impossible for a lab member to intentionally leak the pathogens by oneself.
That being said, there are still some other ways for a virus to get out from the lab.
2.1 Mishandled Lab Animals
One Netizen, Wu Xiaohua, raised the safety issues of lab animals in February 2020, “Some labs have very poor management on this and sell lab animals for profit. For example, dogs were raised as pets (Union Medical College has done this previously) … The dead bodies of lab animals were also handled inappropriately. Instead of paying the costly incineration expense, South Medical University and other places have sold them, including macaques, as wildlife.
“I have seen students in the lab cooking specific pathogen free eggs [for vaccine production] for meals; Lab pigs were slaughtered with meat shared by lab members…. Some took lab mice out in pockets and raised them as pets…
“The mutation and recombination of virus could happen by accident. But the lab management has lots of troubles.”
Because what Wu said was reality and it existed in many Chinese labs, no scientists refuted his message.
The CCP official media did not respond to Wu’s opinions either; it instead said the BSL-4 lab had high security and the negative pressure would prevent accidental leaking.
But if Wuhan Institute of Virology is unable to contain pathogens because of mishandling of lab animals, that alone would endanger its safety measures.
According to policy, lab animals first need disinfection on the surface, being sealed in bags, and kept in freezers. Like other medical infectious waste, they are then sent to an incinerator for two-stage incineration, which is a process of a minimum 4 hours of burn time at 1,800 ºF (about 1,000 ºC).
Wuhan Institute of Virology does not have its own incinerator and sends its lab animals to contractors for incineration, including mice, pigs, sheep, and so on. This is also how lab animals are handled in the Western countries according to medical ethics.
But in the CCP-ruled China, this can be a serious loophole.
People may think, after formalin treatment and freezing, the lab animals would only be burned. In China, however, anything can happen.
Simple searching in Baidu, a popular Chinese search engine, with keywords of “formalin” and “meat” would lead to a large number of websites with information on how to use toxic formalin to preserve meat. More specifically, formalin treatment would make rotten meat, or meat from dead animals, look fresh.
After the addition of meat tenderiser (which in itself could also be poisonous during adulteration with nitrites) and other seasoning, consumers would probably enjoy the flavor without knowing what is in it.
It is apparent that even if lab operators closely follow all procedures, what happens outside the lab is out of their control and may still allow pathogen to infect people with no limitation.
In the above discussions, Wu Xiaohua challenged Shi Zhengli about lab safety. Shi did not respond to this probably because lab animal handling was something outside of her responsibilities.
On February 17, an article appeared on the social media site of Weibo, claiming that Wang Yanyi, director of Wuhan Virology Institute, had leaked pathogens.
“I am Chen Quanjiao, a research fellow at Wuhan Virology Institute and my ID number is 422428197404080626,” read the post, which said that Wang was promoted to her position because of her husband Shu Hongbing, a member of the Chinese Academy of Sciences and dean of Wuhan University Medical School.
“She [Wang] often takes some animals from the lab and sells them to vendors at Huanan Seafood Market,” the post continued.
After this post, however, Chen was silenced and detained by police. It was reported that officials attempted to force her to withdraw those statements publicly on TV. It was unclear whether she complied with the demand.
Chen was later released, but neither she nor her family was willing to talk more about it. “We don’t want to get in trouble,” one family member said.

2.2 System Failure
What Wu Xiaohua and Chen Quanjiao said was supported by numerous evidence. On January 2, 2020, Li Ning, member of Chinese Academy of Sciences in China Agricultural University, was sentenced to 12 years of imprisonment. According the verdict (2015-Songyuan County Criminal Case No. 15), Li embezzled 37.6 million yuan of scientific funds, among which 10.2 million yuan came from selling deserted lab animals and milk. That is, Li sold the genetically modified cows and milk in the lab to consumers and made a fortune.
Neither China Agricultural University nor Wuhan Institute of Virology has effective safety monitoring systems in place. Just like the CCP itself, the self-monitoring process is usually neglected. Only after major issues break out do people know of their existence.
As for what Chen reported, the profit from selling animals is not that much. Among animals in Wuhan Institute of Virology, mice do not sell well, and the numbers of pigs, dogs, sheep, rabbits, or snakes are small (as the lab is different from an agricultural lab). The bigger sum could be the portion of scientific funding that pays for the medical waste processing (including incineration of lab animals).
Some greedy contractors could receive payments from research facilities (like Wuhan Institute of Virology) for incineration and also make money by selling lab animals instead of burning them as specified in their contracts.
After the coronavirus outbreak, Chinese leader Xi Jinping said in February that lab biosafety should be treated as a national security issue. The next day, the Science and Technology Ministry rolled out new regulations via a document titled “Guiding opinions on strengthening biosecurity management in microbiology labs that handle advanced viruses at the same level as the new coronavirus.”
It remains unclear how effective such regulations would be. As described in Part 1 of the series, the CCP had all the resources in place to identify, report, and publicise outbreaks such as coronavirus. But when doctors, scientists, and testing facilities identified coronavirus and reported to higher-ups, they were silenced and punished.
As for the lab animal handling, it involves underlying social responsibility and ethics. It’s also beyond regulations and we will discuss in Part 4.
Unfortunately, as medical professionals were reprimanded for raising awareness of the epidemic, officials ordered a deep cleaning of Huanan Seafood Market on December 31 and it was shut down on January 1.
These series of actions make it difficult to further investigate the wet market, that the Chinese authorities claimed was where the virus first broke out and where some of the dead animals disposed of by the virology lab were sold.
3. Shi-discovered virus infected her team and facilitated later transmission
Another possible transmission route for the virus to be spread from the bat to the unknown intermediate host was through Shi’s team members.
To have a full picture of this possibility, we will have to look at what Shi did after the 2003 SARS outbreak.
3.1 A Look Back at the Discovery of SARS
After the 2003 SARS epidemic, Shi and her team from Wuhan Institute of Virology set out to look for the origin of it, in order to prevent another major epidemic.
They spent seven years and went to many places to look for the virus. They didn’t know whether they would succeed until one day they stopped by a bat cave in Kunming, Yunnan Province.
Through sequencing, Shi was delighted to find that a naturally occurring virus from the horseshoe bat, sharing 97% homology to the SARS virus.
After another five years of sampling and analysis, Shi’s team proved the horseshoe bat species was the source of the SARS virus in a 2013 Nature paper.
Shi proposed the infection route of the 2003 SARS:
SARS virus in bat in Yunan à civet in Yunan (from captive breeding) à Guangdong Province where civets were sold and the virus eventually evolved into SARS-CoV à outbreak in humans.
Shi’s work was recognised by her peers, which earned her the title of “Bat Woman.”
3.2 Possible Infection of Team Members Back Then
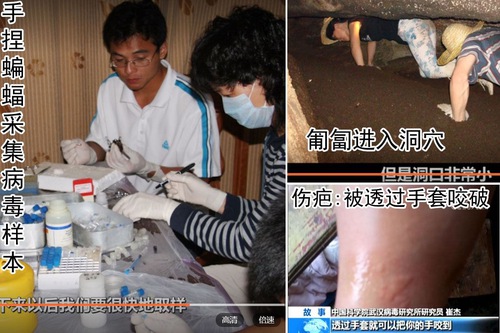
As shown in the above pictures, when Shi’s team spent six years working in Yunnan collecting bat virus samples, they worked in close proximity to bats including taking samples. Some did not wear facial masks or gloves. Even those who wore gloves had been bitten by bats and bled.
Shi explained that only when there were too many bats in a cave and the dust caused breathing difficulty did they put on additional personal protection. “Although bats carry many viruses, their chance of infecting people is minimal,” she said during a lecture in June 2018.
But that could be very risky. The paper from Shi’s team on Virologica Sinica in March 2018 found 3% village residents in the area near a bat cave they worked at had antibodies against coronavirus, which is a clear indication of previous infection. Because antibodies concentration would decrease to below detection level over time, the actual infection rate of the villagers could be higher.
How were they infected?
Some had seen bats flying into the village and one person had handled a dead bat; occasionally, some had been near the cave. If so, Shi’s team went into the cave studying the bats and their chances of being infected could be much higher. It is just that the virus was non-toxic and the infections couldn’t cause illnesses.
To note, the bats in the cave, especially the horseshoe bats, are natural hosts of SARS and other viruses. They carry all kinds of SARS-related genes. As the virus constantly goes through exchanges of genetic information (recombination), it is hard to predict what would come out of there. To some extent, it can be called a Pandora’s Box.
If Shi’s team members were infected by the virus and brought it back to Wuhan, it might also explain why the bat virus RaTG13, brought back by Shi’s team members in a test tube, shares 96.2% sequence identity to the Wuhan virus, the closest strain reported so far.
To some extent, this could also explain why the Wuhan Institute of Virology has reported zero infection cases so far. The staff members could have already developed antibodies from previous exposures to non-pathogenic virus. It works like dairy workers’ contact with cowpox protected them from infection by smallpox.
But would antibody detection of the team members help identify how the disease was transmitted?
It may not help much because when the virus are not active, the antibody level also drops; it increases only when encountering similar viruses.